














DreamWarmer™ Infant Warmer
-
Where is the price?
Pricing is based on shipping location and the number of units ordered. To request a price, please use the Request a Quote button below. A sales representative will respond to you quickly.
Key features of DreamWarmer™
Hypothermia is a silent enemy of newborns even in warm, tropical settings. The incidence of hypothermia is highest in low birth weight or premature infants. These vulnerable newborns are dependent upon an external heat source to keep them warm and at a constant body temperature. Hypothermia can cause hypoglycemia, respiratory distress syndrome, jaundice, and metabolic acidosis.
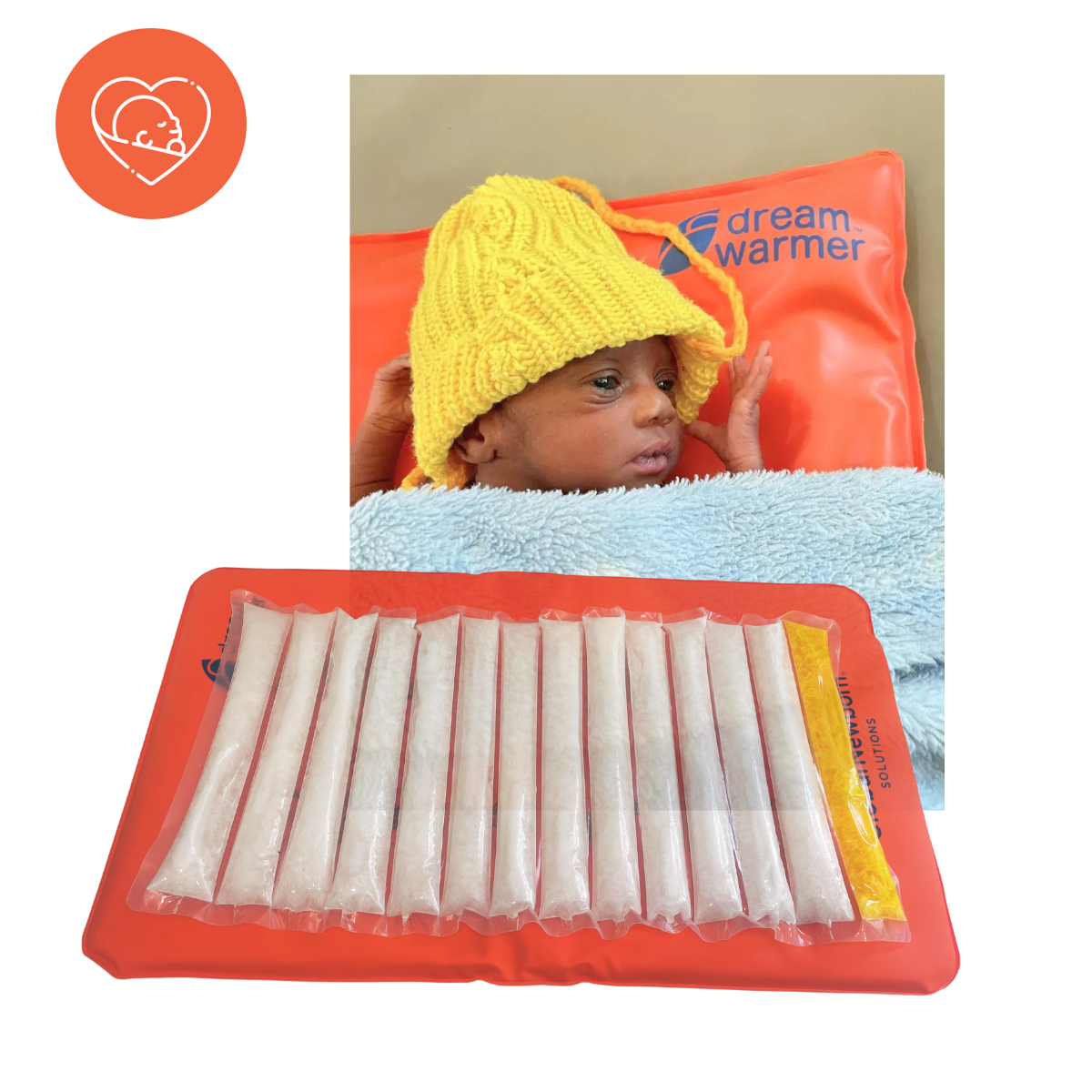
The Durable Re-usable Electricity-free Affordable Mattress called the DreamWarmer™ is a novel, clinically tested solution that allows infant warming on a gel mattress made of phase change material. The material is safe, hygienic and the warmer has been tested with more than 1000 infants. The mattress itself can be heated in hot water (boiled by any means) and can maintain its temperature of 37 degrees C for 6 hours.
The DreamWarmer™ comes with a highly customized thermos which allows for hot water to be readily kept hot for rewarming the device. The DreamWarmer™ can be used alone to warm the infant or wrapper behind the infant with a person providing skin to skin (STS) warming or Kangaroo Mother Care.
Product and ordering information
- English instructions available
- The DreamWarmer™ is sold as a complete kit with the Thermos, bag, 3 mattresses and mattress covers.
- Minimum quantity of 3 kits (9 mattresses and 3 thermoses)
Regulatory approval information
- Meets WHO guidelines for use in low-resource settings
- A ISO 13485 registered company
- The DREAMWarmer™ is not a medical device (letter from the FDA)
- Approved by the Rwanda FDA
FAQs
What are the safety controls in the DreamWarmer™?
This infant warming mattress was designed, piloted and tested over the last decade to ensure that it is safe for infants. There are four key safety features built into the kit.
1. Phase Change Material: Lawrence Berkeley Laboratories innovation
The warming mattress is made of a bio-based phase change material that moves from liquid to solid at 37 °C and once melted it stays consistently at this temperature for approximately six hours.
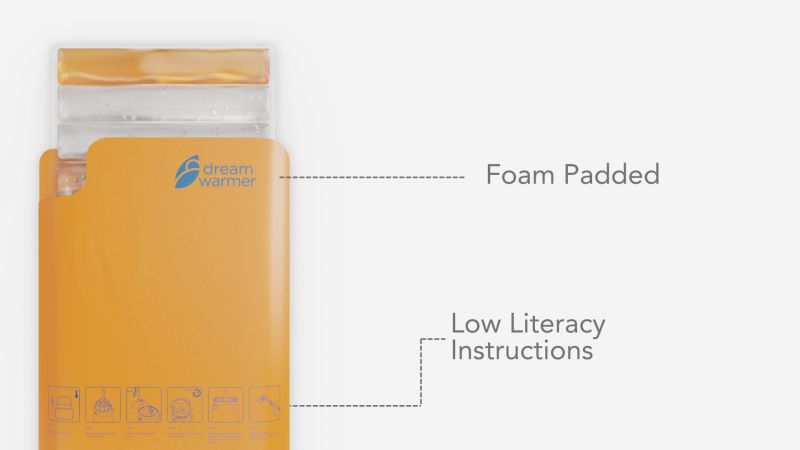
2. Heated Mattress goes inside a neoprene sleeve
The insulating sleeve is a polymer film made of non-toxic foam and neoprene, certified by the USDA and FDA to hold air, fluids, and vacuum, and to withstand very high temperatures. This protects the infant and keeps the mattress warm for over 6 hours. It’s also easy to clean for infection prevention.
3. Bright Orange Warming Indicator
When the ORANGE indicator built into the mattress turns from liquid to solid it is safe to put the baby on the pad.
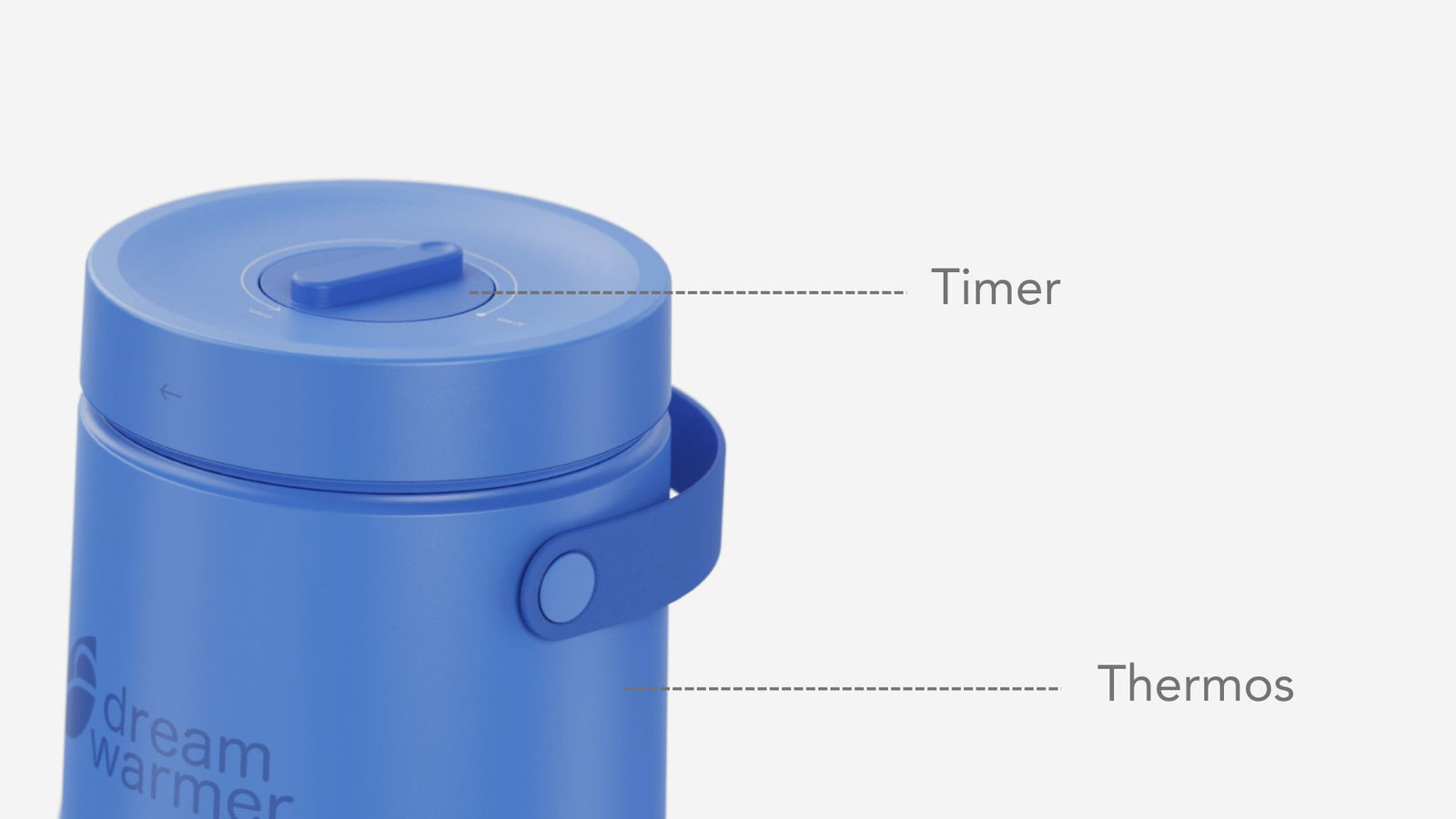
4. Custom-designed Heating Thermos:
The thermos has a wide base so it doesn’t tip over with a lid so it closes. It has a built-in timer to specify the precise amount of time needed to transfer heat from water to phase change material. The precise volume of boiling water is indicated with a filling line inside the thermos.
What peer-reviewed studies back up the use of the DreamWarmer ™ in low-resource hospital settings?
• The DreamWarmer ™ has been studied in two pilot studies (published in Public Health Action, 2018 and Global Pediatric Health, 2019)
• A large, stepped wedge cluster randomized trial in Rwanda published in The Lancet
Implementation science studies in Malawi, Rwanda (accepted for publication), and Chiapas, Mexico (data currently being analyzed)



















