Providence, RI, March 8, 2016 (Newswire.com) - Maternova, Inc , the global public health solutions provider has signed as the exclusive distribution partner for Microlife Corporation’s pathbreaking Vital Signs Alert (VSA). This newest addition to the Maternova women’s health portfolio is positioned for rapid adoption globally.
“The VSA is the first such device validated in pregnant women and for use in emerging market and low resource settings” Maternova’s CEO Meg Wirth shared. “ We’re pleased to be the commercialization partner for this combination blood pressure and heart rate monitor which has exceeded World Health Organization performance requirements,” Wirth added.
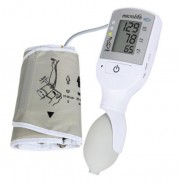
The VSA is a handheld semi-automatic oscillometric device measures vital signs, with a simplified readout based on traffic signal warnings (red, yellow and green lights) for shock and high blood pressure. The device is critical breakthrough to identify shock after hemorrhage and high blood pressure due to preeclampsia or eclampsia. These conditions are responsible for the majority of maternal deaths globally.
Developed in the United Kingdom, by Professor Andrew Shennan, CRADLE Chief Investigator, Kings College London, the Microlife VSA has undergone extensive field-testing multicenter trials in ten low-income sites, and has been lauded as a true breakthrough for expectant mothers at risk. “We are delighted that we are at last in a position to disseminate the device to the areas that need it. It has been a long journey, and taken more than 10 years to get right. But now the new technology is proven to be effective, popular and robust with the potential to save many lives. We just need to get it to where it's needed and Maternova is the perfect partner to achieve this.” Dr. Shennan added.
Microlife is one of the world leaders in the development and manufacturing of medical diagnostic equipment for home monitoring and institutional use. “We are very proud about the newly developed VSA Device and the positive feedback and enthusiasm we received from health professionals working for the Cradle Projects. We deeply hope and believe that with this device we can improve maternal care and reduce maternal deaths in low and middle-income countries” added Mr. A. Moll, Sales Microlife.
Maternova, Inc. is a company specializing in distribution of obstetric and neonatal innovations and solutions for emerging markets.
The Vital Signs Alert device will be available as either an individual product, and as part of Maternova Inc.’s new solution kits for Postpartum Hemorrhage and Safe Delivery. To learn more about the VSA and for efficient ordering, please email orders@maternova.net or also visit online to learn more at this Matrenova Cradle VSA link or the Maternova site.