




Preemie Test
-
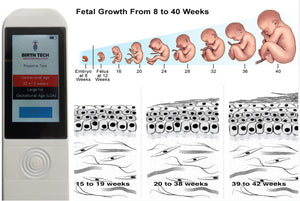
This simple-to-operate, evidence-based device can assess the gestational age of a newborn using light to determine skin maturity (the device detects the preterm newborns by analyzing the photobiological properties of the newborn's skin).
- Gestational age is the major marker of neonatal survival
- Portable and easy for non-specialists to use to assess gestational age and make clinical decisions about transport or resuscitation
- Device warns about errors of measurement
According to the WHO, every year around 15 million babies are born too early and 1 million die due to prematurity complications. Whenever a baby is born too small, birth attendants need to make time-sensitive decisions about immediate interventions and referral of the newborn to a hospital. It is important to immediately determine whether a newborn is preterm or small for gestational age. Without critical care, newborns who are preterm are at risk for a range of short-term mortality and life-long risks. Most of these lives could be saved with prompt neonatal prematurity identification. However, in the absences of an ultrasound (often too expensive and not accessible) there is no gold standard for pregnancy dating.
THE SOLUTIONThe Preemie Test is the first medical device capable of accurately assessing gestational age immediately after an infant is born. The Preemie Test features a probe containing light emitters and receivers that is brought in contact with the newborn’s foot and, using mathematical algorithms, provides an estimated dating within seconds. This noninvasive optoelectronic device measures the maturity (thickness) of the skin through backscattering of light from the skin, using a light emitting diode at wavelengths of 470 nm, 575 nm, and 630 nm. Multiple clinical trials have validated the effectiveness and accuracy of the Preemie Test devices (Brazil, Portugal, Mozambique, India and Malawi). All studies are available upon request
Immediately after childbirth, a newborn with unknown or unreliable gestational age requires resuscitation with initial steps in stabilization and positive-pressure ventilation. Differentiating immediately between SGA and IUGR is critical as is determining the level of prematurity (in weeks) with certainty.
The battery for this device lasts for three years allowing hundreds, or thousands of measurements. According to the Brazilian regulatory health agency (ANVISA), this medical device is categorized as a Class II safety: non-invasive and medium risk.
Where is the price?
Pricing is based on shipping location and the number of units ordered. To request a price, please use the Request a Quote button below. A sales representative will respond to you quickly.
This simple-to-operate, evidence-based device can assess the gestational age of a newborn using light to determine skin maturity (the device detects the preterm newborns by analyzing the photobiological properties of the newborn's skin).
- Gestational age is the major marker of neonatal survival
- Portable and easy for non-specialists to use to assess gestational age and make clinical decisions about transport or resuscitation
- Device warns about errors of measurement
According to the WHO, every year around 15 million babies are born too early and 1 million die due to prematurity complications. Whenever a baby is born too small, birth attendants need to make time-sensitive decisions about immediate interventions and referral of the newborn to a hospital. It is important to immediately determine whether a newborn is preterm or small for gestational age. Without critical care, newborns who are preterm are at risk for a range of short-term mortality and life-long risks. Most of these lives could be saved with prompt neonatal prematurity identification. However, in the absences of an ultrasound (often too expensive and not accessible) there is no gold standard for pregnancy dating.
THE SOLUTIONThe Preemie Test is the first medical device capable of accurately assessing gestational age immediately after an infant is born. The Preemie Test features a probe containing light emitters and receivers that is brought in contact with the newborn’s foot and, using mathematical algorithms, provides an estimated dating within seconds. This noninvasive optoelectronic device measures the maturity (thickness) of the skin through backscattering of light from the skin, using a light emitting diode at wavelengths of 470 nm, 575 nm, and 630 nm. Multiple clinical trials have validated the effectiveness and accuracy of the Preemie Test devices (Brazil, Portugal, Mozambique, India and Malawi). All studies are available upon request
Immediately after childbirth, a newborn with unknown or unreliable gestational age requires resuscitation with initial steps in stabilization and positive-pressure ventilation. Differentiating immediately between SGA and IUGR is critical as is determining the level of prematurity (in weeks) with certainty.
The battery for this device lasts for three years allowing hundreds, or thousands of measurements. According to the Brazilian regulatory health agency (ANVISA), this medical device is categorized as a Class II safety: non-invasive and medium risk.









