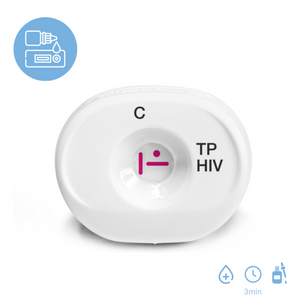


Miriad TP/HIV Rapid Test
DMTSTRH12SY
Multiplo TP/HIV is a rapid test for the simultaneous detection of antibodies to HIV-1/2 and/or syphilis in whole blood, serum, and plasma specimens.
- Employs MedMira Rapid Vertical Flow Technology to deliver the simultaneous diagnosis of syphilis (Treponema pallidum bacteria) and HIV-½
- Five times faster than lateral flow
- Results in less than three minutes
- Built-in reagent control line
- No equipment needed
- Shelf-life 12 months 2-30C or 35.6-86F
- Three easy steps
- Instant Results
- 100% accurate, Virtually 100% sensitivity and specificity for TP and HIV
- Fingerstick-one drop of blood
MedMira's Multiplo TP/HIV Rapid Test: One Test, More Answers
Human immunodeficiency virus (HIV) is a blood-borne virus typically transmitted via sexual intercourse, shared intravenous drugs’ needles, and during the birth process or via human milk (vertical transmission). HIV disease is caused by infection with HIV-1 or HIV-2, which are retroviruses in the Retroviridae family,Lentivirus genus.
Signs and symptoms
The patient with HIV may present with signs and symptoms of any of the stages of HIV infection. No physical findings are specific to HIV infection. Manifestations include the following
- Acute seroconversion manifests as a flulike illness consisting of fever, malaise, and a generalized rash
- The asymptomatic phase generally is benign
- Generalized lymphadenopathy is common and may be a presenting symptom
- AIDS manifests as recurrent, severe, and occasionally life-threatening infections or opportunistic malignancies
- HIV infection can cause AIDS-associated dementia/encephalopathy and HIV wasting syndrome (chronic diarrhea and weight loss with no identifiable cause)
An estimated 430,000 children were newly infected with HIV in 2008, over 90% of them through mother-to-child transmission (MTCT). Syphilis is a sexually transmitted disease caused by the bacterium Treponema pallidum (TP). Mother-to-child transmission (MTCT) of syphilis is a major cause of adverse pregnancy outcomes. Syphilis and HIV share the common routes of transmission, thus the majority of individuals considered at risk of infection with one of the diseases are consequently at risk with the other one.
The US Preventive Services Task Force (USPSTF) recommends that clinicians screen for HIV in all adolescents and adults at increased risk for HIV infection, and all pregnant individuals.
Syphilis is a sexually transmitted infection caused by the bacterium (spirochete)Treponema pallidum.T pallidum is solely a human pathogen and does not naturally occur in other species. It is primarily spread through sexual contact but can be spread by exposure to blood products and transferred in utero.T pallidum cannot survive drying or exposure to disinfectants; thus, fomite transmission (eg, from toilet seats) is virtually impossible.
After an incubation period of 10 to 90 days (three weeks on average), clinical symptoms appear: the first is a primary lesion at the site of infection (chancre), then a series of eruptions on mucous membranes and skin (secondary syphilis), followed by long periods of latency (latent or tertiary syphilis). If untreated, many years after the initial infection, tertiary syphilis lesions might finally appear (visceral, multi-organ involvement, including serious vascular and neurological damage). Mother-to-child transmission might result in fetal death, perinatal death or congenital syphilis. The latter can be without symptoms or present with stigmata or multi-organ pathology. With the widespread use of penicillin, syphilis prevalence had significantly declined after World War II. However, in several industrialized countries, a considerable resurgence occurred in late 1980.
FAQs
Why is it important to test for both syphilis and HIV?
Because they are both sexually transmitted diseases that can adversely affect both mother and baby and both diseases can be treated. HIV cannot be cured but drugs can slow the progression. However, syphilis can be cured and is best treated early to prevent complications of pregnancy and for mothers because advanced syphilis can be hard to treat and has serious complications.
What is the Test’s INTENDED USE?
Multiplo TP/HIV is a single‐use, qualitative immunoassay for the detection of antibodies to Treponema pallidum bacteria (TP), the causative agent of syphilis and/or antibodies to human immunodeficiency virus Type 1 and Type 2 (anti‐HIV‐1/2) in human serum, plasma, and whole blood (fingerstick and venipuncture). Multiplo TP/HIV is intended for use by healthcare professionals as an aid in the diagnosis of infection with TP and/or HIV‐1/2.
How does the TEST Work?
Multiplo TP/HIV is a manually performed, visually interpreted, rapid vertical flow immunoassay.
The test cartridge contains an immunoreactive test membrane comprised of specially formulated synthetic peptides coated onto a membrane matrix, which functions to capture anti‐TP and anti‐HIV‐1/2 antibodies present in human serum, plasma and whole blood when a drop of the specimen is applied.
What are the contents?
(Fingerstick Whole Blood) Includes a test tray in each pouch
20 Mylar pouches each containing:1 test cartridge
1 InstantGold cap
1 silica gel packet
1 auto‐fill pipelle
1 Universal Buffer vial 1
1 Universal Buffer vial 2
1 lancet (sterile)
1 alcohol swab
1 package insert
1 lancet use card
Instructions for Use
Fingerstick Whole Blood Specimens
SPECIMEN HANDLING & COLLECTION
- Uncap and place Universal Buffer vial 1 into the hole of the test tray.
- Using an alcohol swab, clean the finger. Allow the finger to dry thoroughly.
- Using the lancet provided with the test, lance the fingertip in preparation for collection of the fingerstick whole blood specimen.
- Use the auto‐fill pipette provided to collect a drop of blood from the fingerstick site. To do this, hold the pipelle horizontally and touch the drop of blood. The blood sample will be automatically drawn to the black fill line and stop. Do not squeeze the pipe tte bulb during filling.
- Place the tip of the pipe tte into the Universal Buffer in Universal Buffer vial 1. Squeeze the bulb to empty the blood sample into the vial. Recap Universal Buffer vial.
- Hold Universal Buffer vial 1 and gently tap the side of the vial near the bottom until the mixture becomes a clear reddish color. This can take 15‐30 seconds of gentle tapping to mix properly.
TEST PROCEDURE
- Pour the entire contents of Universal Buffer vial 1 into the center of the test cartridge. Allow the solution to absorb completely.
- Place the InstantGold cap on the test cartridge.
- Pour the entire contents of Universal Buffer vial 2 onto the InstantGold cap and allow the solution to absorb completely.
- Remove the InstantGold cap and wait for the solution to absorb completely. Read test results immediately.
References
- Justiz Vaillant AA, Naik R. HIV-1 Associated Opportunistic Infections.StatPearls. 2022 Jan.
- Waymack JR, Sundareshan V. Acquired Immune Deficiency Syndrome.StatPearls. 2022 Jan.
- US Preventive Services Task Force., Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Screening for HIV Infection: US Preventive Services Task Force Recommendation Statement.JAMA. 2019 Jun 18. 321 (23):2326-2336.
- Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings.MMWR Recomm Rep. 2006 Sep 22. 55:1-17; quiz CE1-4.
- Qaseem A, Snow V, Shekelle P, Hopkins R Jr, Owens DK. Screening for HIV in health care settings: a guidance statement from the American College of Physicians and HIV Medicine Association.Ann Intern Med. 2009 Jan 20. 150(2):125-31.
- MedMira's Multiplo TP/HIV Rapid Test: One Test, More Answers.
- CESIPYZIS0001EN Multiplo Rapid TP HIV Test Package Insert.pub (medmira.com)
Where is the price?
Pricing is based on shipping location and the number of units ordered. To request a price, please use the Request a Quote button below. A sales representative will respond to you quickly.
Multiplo TP/HIV is a rapid test for the simultaneous detection of antibodies to HIV-1/2 and/or syphilis in whole blood, serum, and plasma specimens.
- Employs MedMira Rapid Vertical Flow Technology to deliver the simultaneous diagnosis of syphilis (Treponema pallidum bacteria) and HIV-½
- Five times faster than lateral flow
- Results in less than three minutes
- Built-in reagent control line
- No equipment needed
- Shelf-life 12 months 2-30C or 35.6-86F
- Three easy steps
- Instant Results
- 100% accurate, Virtually 100% sensitivity and specificity for TP and HIV
- Fingerstick-one drop of blood
MedMira's Multiplo TP/HIV Rapid Test: One Test, More Answers
Human immunodeficiency virus (HIV) is a blood-borne virus typically transmitted via sexual intercourse, shared intravenous drugs’ needles, and during the birth process or via human milk (vertical transmission). HIV disease is caused by infection with HIV-1 or HIV-2, which are retroviruses in the Retroviridae family,Lentivirus genus.
Signs and symptoms
The patient with HIV may present with signs and symptoms of any of the stages of HIV infection. No physical findings are specific to HIV infection. Manifestations include the following
- Acute seroconversion manifests as a flulike illness consisting of fever, malaise, and a generalized rash
- The asymptomatic phase generally is benign
- Generalized lymphadenopathy is common and may be a presenting symptom
- AIDS manifests as recurrent, severe, and occasionally life-threatening infections or opportunistic malignancies
- HIV infection can cause AIDS-associated dementia/encephalopathy and HIV wasting syndrome (chronic diarrhea and weight loss with no identifiable cause)
An estimated 430,000 children were newly infected with HIV in 2008, over 90% of them through mother-to-child transmission (MTCT). Syphilis is a sexually transmitted disease caused by the bacterium Treponema pallidum (TP). Mother-to-child transmission (MTCT) of syphilis is a major cause of adverse pregnancy outcomes. Syphilis and HIV share the common routes of transmission, thus the majority of individuals considered at risk of infection with one of the diseases are consequently at risk with the other one.
The US Preventive Services Task Force (USPSTF) recommends that clinicians screen for HIV in all adolescents and adults at increased risk for HIV infection, and all pregnant individuals.
Syphilis is a sexually transmitted infection caused by the bacterium (spirochete)Treponema pallidum.T pallidum is solely a human pathogen and does not naturally occur in other species. It is primarily spread through sexual contact but can be spread by exposure to blood products and transferred in utero.T pallidum cannot survive drying or exposure to disinfectants; thus, fomite transmission (eg, from toilet seats) is virtually impossible.
After an incubation period of 10 to 90 days (three weeks on average), clinical symptoms appear: the first is a primary lesion at the site of infection (chancre), then a series of eruptions on mucous membranes and skin (secondary syphilis), followed by long periods of latency (latent or tertiary syphilis). If untreated, many years after the initial infection, tertiary syphilis lesions might finally appear (visceral, multi-organ involvement, including serious vascular and neurological damage). Mother-to-child transmission might result in fetal death, perinatal death or congenital syphilis. The latter can be without symptoms or present with stigmata or multi-organ pathology. With the widespread use of penicillin, syphilis prevalence had significantly declined after World War II. However, in several industrialized countries, a considerable resurgence occurred in late 1980.
FAQs
Why is it important to test for both syphilis and HIV?
Because they are both sexually transmitted diseases that can adversely affect both mother and baby and both diseases can be treated. HIV cannot be cured but drugs can slow the progression. However, syphilis can be cured and is best treated early to prevent complications of pregnancy and for mothers because advanced syphilis can be hard to treat and has serious complications.
What is the Test’s INTENDED USE?
Multiplo TP/HIV is a single‐use, qualitative immunoassay for the detection of antibodies to Treponema pallidum bacteria (TP), the causative agent of syphilis and/or antibodies to human immunodeficiency virus Type 1 and Type 2 (anti‐HIV‐1/2) in human serum, plasma, and whole blood (fingerstick and venipuncture). Multiplo TP/HIV is intended for use by healthcare professionals as an aid in the diagnosis of infection with TP and/or HIV‐1/2.
How does the TEST Work?
Multiplo TP/HIV is a manually performed, visually interpreted, rapid vertical flow immunoassay.
The test cartridge contains an immunoreactive test membrane comprised of specially formulated synthetic peptides coated onto a membrane matrix, which functions to capture anti‐TP and anti‐HIV‐1/2 antibodies present in human serum, plasma and whole blood when a drop of the specimen is applied.
What are the contents?
(Fingerstick Whole Blood) Includes a test tray in each pouch
20 Mylar pouches each containing:1 test cartridge
1 InstantGold cap
1 silica gel packet
1 auto‐fill pipelle
1 Universal Buffer vial 1
1 Universal Buffer vial 2
1 lancet (sterile)
1 alcohol swab
1 package insert
1 lancet use card
Instructions for Use
Fingerstick Whole Blood Specimens
SPECIMEN HANDLING & COLLECTION
- Uncap and place Universal Buffer vial 1 into the hole of the test tray.
- Using an alcohol swab, clean the finger. Allow the finger to dry thoroughly.
- Using the lancet provided with the test, lance the fingertip in preparation for collection of the fingerstick whole blood specimen.
- Use the auto‐fill pipette provided to collect a drop of blood from the fingerstick site. To do this, hold the pipelle horizontally and touch the drop of blood. The blood sample will be automatically drawn to the black fill line and stop. Do not squeeze the pipe tte bulb during filling.
- Place the tip of the pipe tte into the Universal Buffer in Universal Buffer vial 1. Squeeze the bulb to empty the blood sample into the vial. Recap Universal Buffer vial.
- Hold Universal Buffer vial 1 and gently tap the side of the vial near the bottom until the mixture becomes a clear reddish color. This can take 15‐30 seconds of gentle tapping to mix properly.
TEST PROCEDURE
- Pour the entire contents of Universal Buffer vial 1 into the center of the test cartridge. Allow the solution to absorb completely.
- Place the InstantGold cap on the test cartridge.
- Pour the entire contents of Universal Buffer vial 2 onto the InstantGold cap and allow the solution to absorb completely.
- Remove the InstantGold cap and wait for the solution to absorb completely. Read test results immediately.
References
- Justiz Vaillant AA, Naik R. HIV-1 Associated Opportunistic Infections.StatPearls. 2022 Jan.
- Waymack JR, Sundareshan V. Acquired Immune Deficiency Syndrome.StatPearls. 2022 Jan.
- US Preventive Services Task Force., Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Screening for HIV Infection: US Preventive Services Task Force Recommendation Statement.JAMA. 2019 Jun 18. 321 (23):2326-2336.
- Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings.MMWR Recomm Rep. 2006 Sep 22. 55:1-17; quiz CE1-4.
- Qaseem A, Snow V, Shekelle P, Hopkins R Jr, Owens DK. Screening for HIV in health care settings: a guidance statement from the American College of Physicians and HIV Medicine Association.Ann Intern Med. 2009 Jan 20. 150(2):125-31.
- MedMira's Multiplo TP/HIV Rapid Test: One Test, More Answers.
- CESIPYZIS0001EN Multiplo Rapid TP HIV Test Package Insert.pub (medmira.com)




